Brownian motion
In a solid material, the attractive forces are strong enough that the atoms or molecules move only slightly (oscillate) about relatively fixed positions.
 In a liquid, the atoms or molecules are moving more rapidly, or the forces between them are weaker, so that they are sufficiently free to pass over one another.
In a liquid, the atoms or molecules are moving more rapidly, or the forces between them are weaker, so that they are sufficiently free to pass over one another.
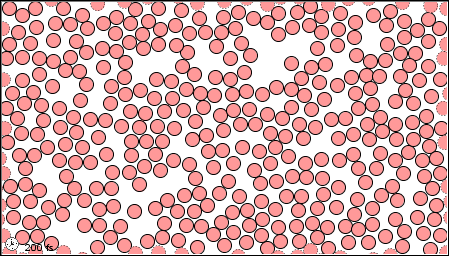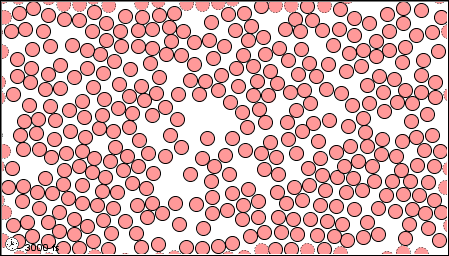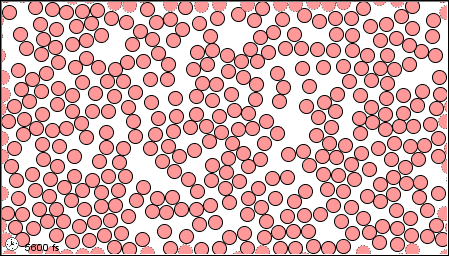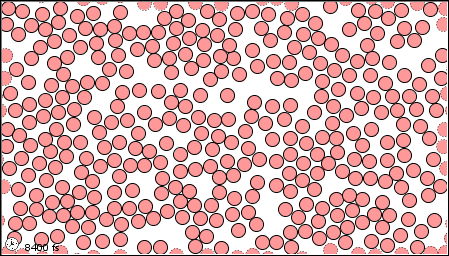 In a gas, the forces are so weak, or thee speeds so high, that the molecules do not even stay close together.
In a gas, the forces are so weak, or thee speeds so high, that the molecules do not even stay close together.
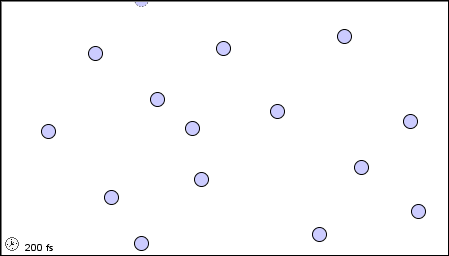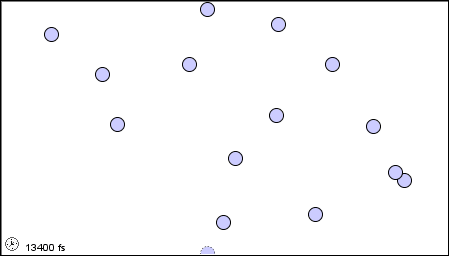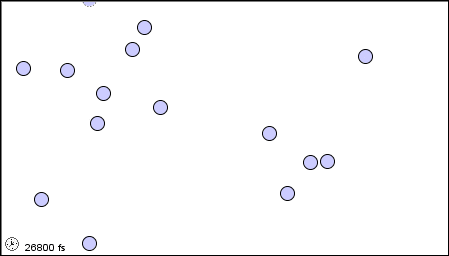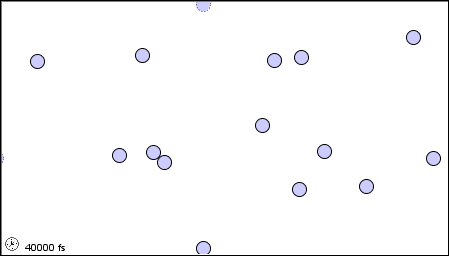
 In a liquid, the atoms or molecules are moving more rapidly, or the forces between them are weaker, so that they are sufficiently free to pass over one another.
In a liquid, the atoms or molecules are moving more rapidly, or the forces between them are weaker, so that they are sufficiently free to pass over one another.
 In a gas, the forces are so weak, or thee speeds so high, that the molecules do not even stay close together.
In a gas, the forces are so weak, or thee speeds so high, that the molecules do not even stay close together.

