Force from Molecules
Recall: Newton's 2nd Law
`F = (∆P)/(∆t) = (∆(mv))/(∆t)`
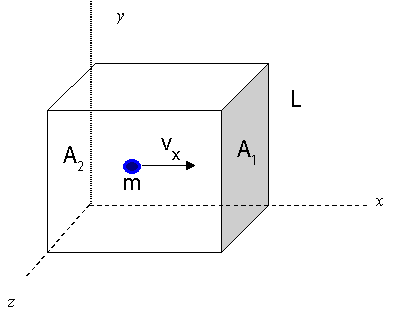 Let consider only one molecule of a gas moving in a rectangular container. Assuming the collision is elastic, only the `x` component of the molecule's momentum changes.
`∆P = mv_x - (-mv_x) = 2mv_x`
`=>`
Let consider only one molecule of a gas moving in a rectangular container. Assuming the collision is elastic, only the `x` component of the molecule's momentum changes.
`∆P = mv_x - (-mv_x) = 2mv_x`
`=>`
`F = (2mv_x)/(∆t)`
Time between 2 collisions:
`∆t = (2L)/v_x`
Then we get
`F = (2mv_x)/((2L)/v_x) = (mv_x^2)/L`
Now let consider huge numbers (N) of molecules of gas moving in a rectangular container, and the average velocity of these molecules is `overline(v)`.
`F = N(moverline(v_x^2))/L`
velocity is a 2D vector `vec(v) = v_xvec(i) + v_xvec(j) +v_zvec(k)`
the magnitude of `v = sqrt(v_x^2 +v_y^2+ v_z^2)`
or `v^2 = v_x^2 +v_y^2+ v_z^2`
Since average velocity `overline(v)` is an average of a huge number. `overline(v_x) = overline(v_y) =overline(v_z)`. we get:
`v^2 = 3v_x^2` or `v_x^2 = 1/3 v^2`
`F = N(moverline(v^2))/(3L)`
