The Gas Laws and Absolute Temperature
Boyle's law:
For a given quantity of gas, the volume of a gas is inversely proportional to the absolute pressure applied to it when the temperature is kept constant.
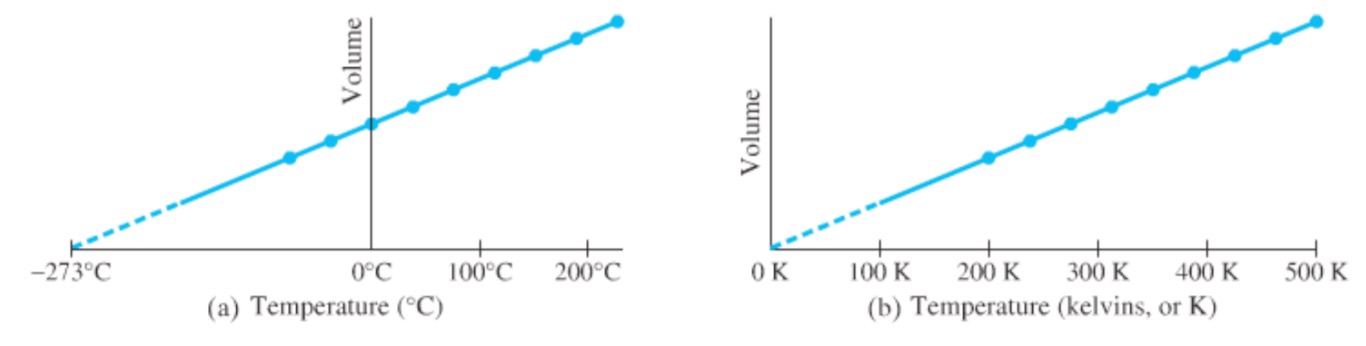 For any gas, the volume and temperature graph is a straight line and always projects back to -273°C, at zero volume. this temperature is called the absolute zero
Absolute zero forms the basis of a temperature scale known as absolute scale or Kelvin scale
For any gas, the volume and temperature graph is a straight line and always projects back to -273°C, at zero volume. this temperature is called the absolute zero
Absolute zero forms the basis of a temperature scale known as absolute scale or Kelvin scale
`PV =` constant, when temperature keeps same.
Charles's law:
For a given quantity of gas, the volume of a gas is directly proportional to the absolute temperature when the pressure is kept constant.
Gay-Lussac's law:
At constant volume, the absolute pressure of a gas is directly proportional to the absolute temperature.
 For any gas, the volume and temperature graph is a straight line and always projects back to -273°C, at zero volume. this temperature is called the absolute zero
Absolute zero forms the basis of a temperature scale known as absolute scale or Kelvin scale
For any gas, the volume and temperature graph is a straight line and always projects back to -273°C, at zero volume. this temperature is called the absolute zero
Absolute zero forms the basis of a temperature scale known as absolute scale or Kelvin scale
`T(K) = T(°C) + 273.15`
